NF-κB drives acquired resistance to a novel mutant-selective EGFR inhibitor
Galvani E, Sun J, Leon LG, Sciarrillo R, Narayan RS, Sjin RTT, Lee K, Ohashi K, Heideman DAM, Alfieri RR, Heynen GJ, Bernards R, Smit EF, Pao W, Peters GJ, Giovannetti E.
Oncotarget 2015 Apr 29. [Epub ahead of print]
Abstract
The clinical efficacy of EGFR tyrosine kinase inhibitors (TKIs) in non-small cell lung cancer (NSCLC) harbouring activating EGFR mutations is limited by the emergence of acquired resistance, mostly ascribed to the secondary EGFR-T790M mutation. Selective EGFR-T790M inhibitors have been proposed as a new, extremely relevant therapeutic approach. Here, we demonstrate that the novel irreversible EGFR-TKI CNX-2006, a structural analog of CO-1686, currently tested in a phase-1/2 trial, is active against in vitro and in vivo NSCLC models expressing mutant EGFR, with minimal effect on the wild-type receptor. By integration of genetic and functional analyses in isogenic cell pairs we provide evidence of the crucial role played by NF-κB1 in driving CNX-2006 acquired resistance and show that NF-κB activation may replace the oncogenic EGFR signaling in NSCLC when effective and persistent inhibition of the target is achieved in the presence of the T790M mutation. In this context, we demonstrate that the sole, either genetic or pharmacologic, inhibition of NF-κB is sufficient to reduce the viability of cells that adapted to EGFR-TKIs. Overall, our findings support the rational inhibition of members of the NF-κB pathway as a promising therapeutic option for patients who progress after treatment with novel mutant-selective EGFR-TKIs.
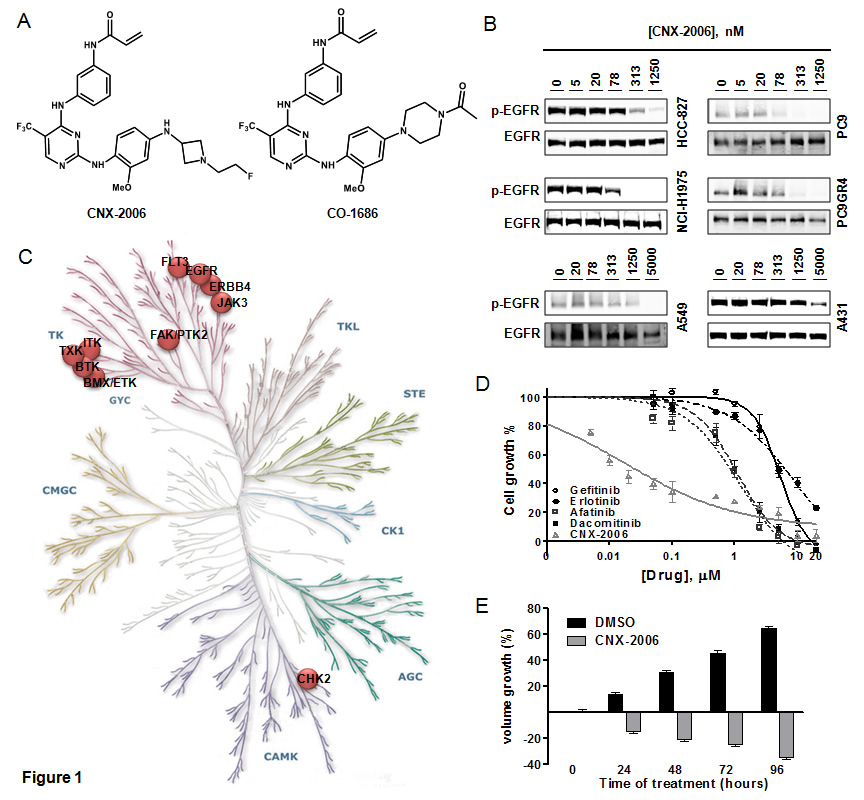
In vitro activity of CNX-2006. A. Molecular structure of CNX-2006 and CO-1686; B. EGFR phosphorylation inhibition evaluated after 2 hours treatment with 0.1% DMSO or the indicated concentrations of CNX-2006; C. kinase inhibition profile of 1 µM CNX-2006 in the presence of 100 µM ATP. The dots indicate enzymes that were inhibited >50% by the inhibitor relative to DMSO. Adjusted from www.cellsignal.com/reference/kinase/index.html; D. anti-proliferative effect of erlotinib (●), gefitinib (○), afatinib (■), dacomitinib (□) and CNX-2006 (∆) in PC9DR1 cells. Data plotted as mean ± SEM; E. effect of 0.1% DMSO or 1 µM CNX-2006 in NCI-H1975-derived tumor spheres. The bar graph shows the mean ± SEM of the percentage of spheroids volume growth normalized to the volume at the time 0 treatment.
Link: www.impactjournals.com/oncotarget
Free full PDF: click here




